Anode and Cathode in Electrolysis
The basic principle of electrolysis is to split water into oxygen and hydrogen with the help of electricity. Most electrolysis problems are really stoichiometry problems with the addition of an amount of electric current.

Physical Chemistry Positive Or Negative Anode Cathode In Electrolytic Galvanic Cell Chemistry Stack Electrochemistry Chemistry Classroom Teaching Chemistry
JES is the flagship journal of The Electrochemical Society.

. The ions are forced to undergo either oxidation at the anode or reduction at the cathode. Both the negative electrode cathode and positive electrode anode are made of graphite which is a form of carbon. Electrolytic cells are often used to decompose chemical compounds in a process called electrolysisthe Greek word lysis means to break upImportant examples of electrolysis are the decomposition of water into hydrogen and oxygen and bauxite into aluminium and other chemicals.
Here electrons are released from the electrode and the surrounding solution is reduced. The chemical change is one in which the substance loses or gains an electron oxidation or reduction. When studying electrodes there are a few things that we come across.
Before we learn about the terms cathode and anode it is important to understand what an electrode is. Electroplating eg of copper silver nickel or chromium is done using an. The slideshow shows what happens.
Published continuously from 1902 to the present JES remains one of the most highly-cited journals in electrochemistry and solid-state science and technology. The process of electrolysis sees electrons being stripped from the anode. The electrolysis takes place at a much lower potential than pure water 24V.
Twice as much hydrogen. The anode consists of an unrefined sample of the metal. Half reactions of electrolysis in the presence of a base are-.
The less noble metal in a reaction will be the anode. Electrolyser electrolysis cell consists of two electrodes called cathode and anode. Then they head towards the cathode.
The quantities of. Water electrolysis relies on the application of an electric current through an anode and cathode inserted in the electrolyte to provide the required energy to break the hydrogen and oxygen bond. So what does that mean.
Water Electrolysis in the Presence of a Base pH higher than 7 Additional hydroxyl ions release their electrons to anode while electrons at the cathode oxidize water molecules near it. The H ions called cations move toward the cathode negative electrode and the OH- ions called anions move toward the anode positive electrode. The process is carried out in an electrolytic cell an apparatus consisting of positive and negative electrodes held apart and dipped into a solution containing positively and.
During electrolysis the anode loses mass as copper dissolves and the cathode gains mass as copper is deposited. While the basis remains the same different electrolysis methods including polymer electrolyte membrane electrolyzer alkaline electrolyzer and solid. A cathode is a negatively charged.
The cathode of this type of cell is made of negatively charged phosphate anions GLOSSARY anions Negatively charged ions especially the ions that migrate to the anode in electrolysis bonded with positively charged iron cations GLOSSARY cations Positively charged ions that is those that would be attracted to the cathode in electrolysis in a structure that is. These catalysts are highly dispersible into inks and in round robin tests came out top in performance and durability evaluations. Electrolysis process by which electric current is passed through a substance to effect a chemical change.
At the cathode hydrogen ions combine with electrons from the external circuit to form hydrogen gas. Low-temperature CO2 electrolysis represents a potential enabling process in the production of renewable chemicals and fuels notably carbon monoxide formic acid ethylene and ethanol. Electrolysis involves passing an electric current through either a molten salt or an ionic solution.
A cathode is a negatively charged electrode. 4H 4e- 2H 2. Alkaline water electrolysis AWE is the most mature and simplest method.
The electrons flow through an external circuit and the hydrogen ions selectively move across the PEM to the cathode. Beijing SinoHy Energy Co Ltd. According to the general definition an electrode is a substance that helps in electricity conduction wherein the electric current either leaves or enters the non-metallic medium such as an electrolytic cell.
The electrolysis of water requires the input of electrical energy and heat energy resulting in the evolution from water of hydrogen gas at the cathode and oxygen gas at the anode according to. The new formed chemical energy can be utilised as a fuel or converted back to electricity when required. The two common terms we hear is cathode and anode.
The largest electrolyzers are of this type and have the greatest commercial reach Dincer and Acar 2014The cell consists of a pair of electrodes separated by a diaphragm that is filled with an alkaline solution typically potassium hydroxide in a concentration between 25 and 30. How does electrolysis work. The dotted vertical line in the above figure represents a diaphragm that prevents the Cl 2 produced at the anode in this cell from coming into contact with the NaOH that accumulates at the cathode.
The splitting occurs in two partial reactions that take place at the two electrodes cathode - and anode in the electrolysis cell. For PEM water electrolyser applications we offer customers making catalyst coated membranes CCMs both anode catalysts IrO 2 and IrRuO 2 and cathode catalysts PtC. The more noble metal in the reaction will always be the cathode.
The diagram shows an aluminium oxide electrolysis cell. With more than ten years development Beijing SinoHy Energy is now an industry-leading hydrogen energy solution provider. The cathode is the current that leaves the electrodes or cathode is a result of reduction reaction taking place in an electrolyte mixture.
In practice electrolysers consist of several interconnected. Our water electrolysis catalyst powders. The cathode is made of pure copper or a support metal such as stainless steel.
The electrolysis can be done using two weighed copper strips. Bubbles of oxygen gas O form at the anode and bubbles of hydrogen gas H 2 2. When this diaphragm is removed from the cell the products of the electrolysis of aqueous sodium chloride react to form sodium hypo-chlorite which is the first step in the.
The anode is a positively charged electrode. Electrolysis transforms electrical energy into chemical energy by storing electrons in the form of stable chemical bonds. This is to confirm that the mass gained at the cathode is.
The bubbles are easily seen. To flow when a voltage is applied. Form at the cathode.
2H 2 O O 2 4H 4e-Cathode Reaction.
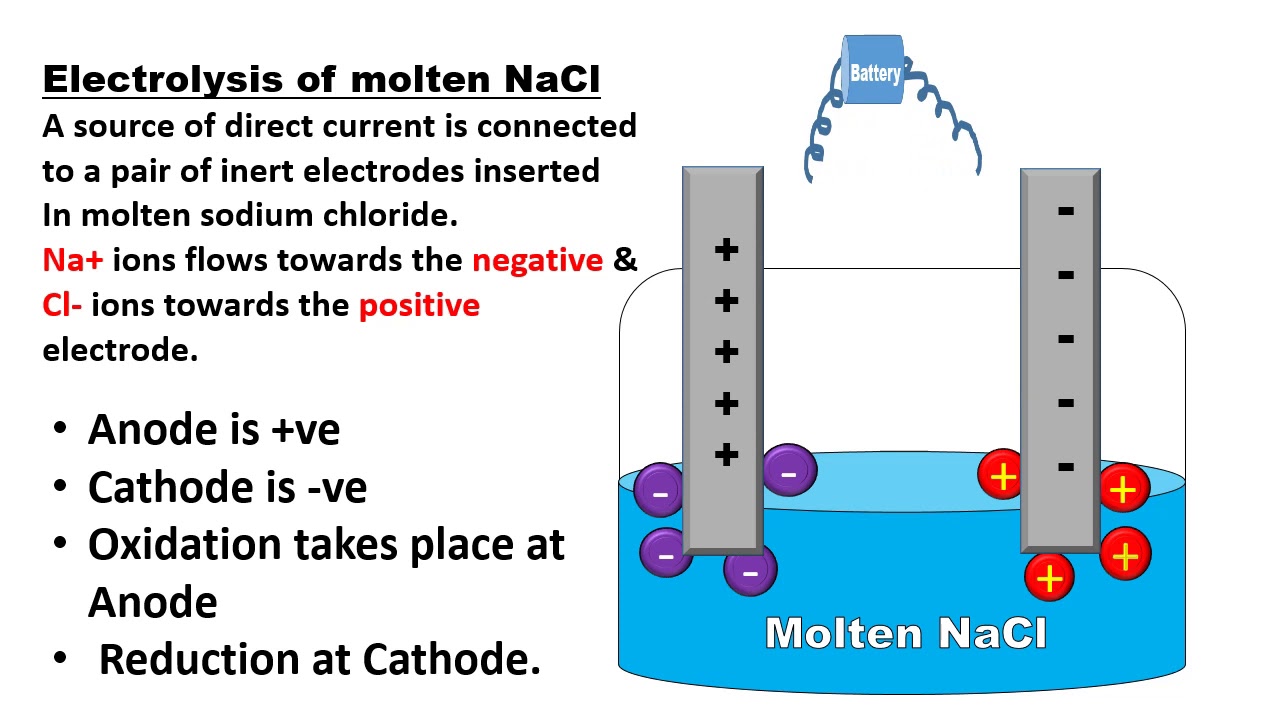
What Is Electrolytic Cell Electrochemistry Chemistry Basics Chemistry

Electrolysis Process On Passing Electric Current The Cations Move Towards The Cathode And Get Deposited Piscinas De Agua Salada Piscinas Escuela De Natacion

Look4chemistry Electrolytic Cell Electrochemistry Cell Redox Reactions

Electrolysis Of Copper Sulfate Solution With Impure Copper Anode And Pure Copper Cathode Copper Purification Technology Educat Pure Products Copper Solutions
Comments
Post a Comment